
Phenol – an important aromatic alcohol with many applications Thiols Isopropanol – this is the rubbing alcohol Methanol – important organic solvent, very toxic, causes blindness If instead of the halogen, we put an OH (hydroxyl group) on an alkyl halide, we get an alcohol.įor the general formula, we have R-OH and below are some common examples: So, alkyl halides have a general formula of R-X, where X can be any halogen atom.Īlkyl groups together with the -CN (cyano) group make the nitriles:Īcetonitrile is a common solvent in organic laboratories and many other nitriles are used in synthetic polymers and glues. This R is what we call an alkyl group, which by itself is shown with a line on the position where we removed the hydrogen: The hydrocarbon chain is often represented with letter R to keep the structure short. If we draw any alkane and remove one of the hydrogens, this brings the possibility of placing other atoms or groups on the position. Now, let’s switch to other functional groups that contain heteroatoms (any atom except carbon and hydrogen). The definition of aromatic compounds is more complex, so for now just remember the benzene ring. Notice that not every cyclic compound with a double bond(s) is aromatic – it must have a certain number of alternating π bonds.įor example, a ring with one double bond is a cycloalkene: Here are some important molecules that contain aromatic rings: What are the key patterns to recognize here? First, remember that it is cyclic (all the aromatic compounds are cyclic), and it has a double bond on every second carbon. However, when learning the functional groups, in most organic 1 classes you would only need to recognize the most common aromatic molecule which is the benzene: This is a big class of compounds with specific physical and chemical properties and there is a different chapter dedicated to studying all of that. They are named just like the alkanes by adding the cyclo– prefix: One important difference is the change of the general formula to C nH 2n as there are no terminal CH 3 groups: The hybridization of triple-bonded carbons is sp which corresponds to the liner geometry – 180 o:Ĭycloalkanes have the same hybridization as the alkanes and only single bonds are present in the molecule. The ending changes from -yne:Īlkynes have two hydrogens less than the corresponding alkenes and therefore the general formula is C nH 2n-2 The difference between alkynes and alkenes is the change of the double bond to a triple bond. The presence of a double bond decreases the number of hydrogens per carbon atom and the general formula changes from C nH 2n+2 to C nH 2n. The double bond is made of one σ and one π bond.

The two carbons with the double bond are sp 2–hybridized, and the geometry is trigonal planar with a 120 o angle between the atoms. This double bond changes the suffix from -ane to -ene. The R groups can be H or hydrocarbon chains (see below about the alkyls) For every n carbon, the molecule has 2n+2 hydrogens.įor example, propane has 3 carbons, therefore it has 2 x 3 + 2 = 8 hydrogens – C 3H 8.Īlkenes are similar to alkanes with the only difference being the presence of a double bond. It will also be helpful to know the general formula of alkanes, which is C nH 2n+2.
Key features: all the carbons are sp 3-hybridized, only single bonds, all the bonds are non-polar, therefore, alkanes and all the other hydrocarbons are non-polar, hydrophobic molecules. You must know the first ten alkanes and their names as they are in the basis of naming and understanding the structure of any other functional groups.

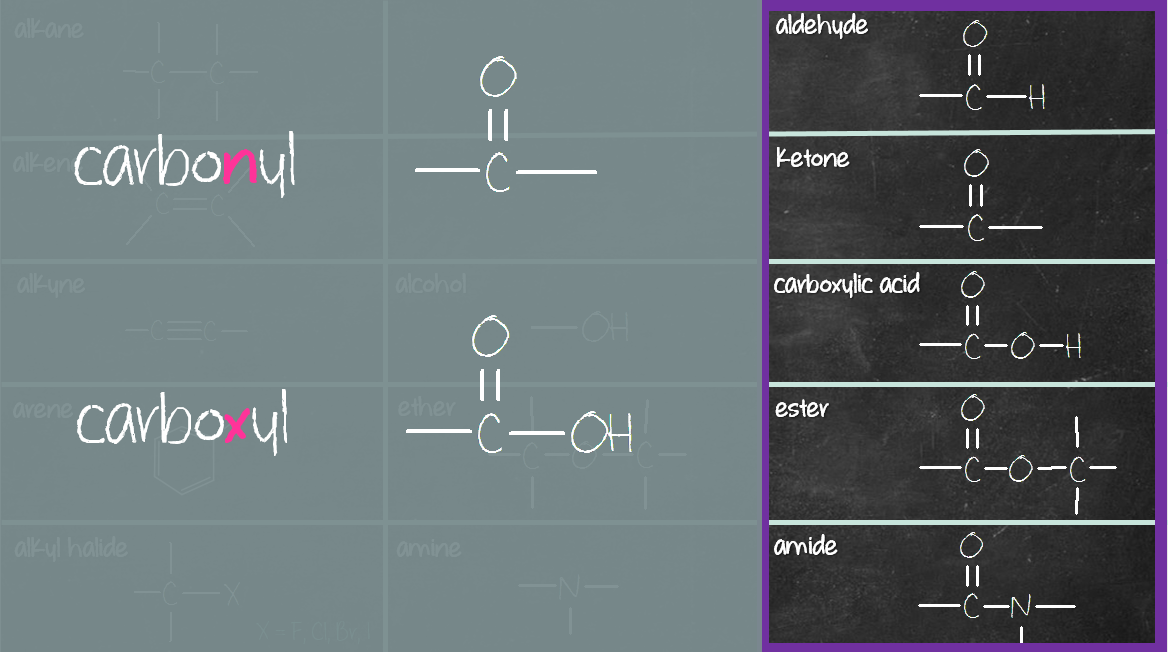
They are made of carbons and hydrogens that are only connected with single ( σ ) bonds. And depending on the connectivity and bonding types, we divide them into five main categories:Īlkanes, alkenes, alkynes, cycloalkanes and aromatic compounds.Īlkanes are the first group of organic compounds. These, as the name suggests, are compounds that consist of carbon and hydrogen atoms. Let’s start from the simplest class of organic compounds – the hydrocarbons. Knowing the functional groups is a must in organic chemistry and in this post, we will go over the structure and interesting applications of the most common functional groups in organic chemistry. A functional group is an atom or a group of atoms that are responsible for the physical and chemical properties of the molecule.


 0 kommentar(er)
0 kommentar(er)
